UCT Biobank
The UCT Biobank was founded in 2009 as a central sample collection of the University Cancer Center (UCT) Frankfurt, unifying preparation and storage of cancer patient samples. The following clinics, institutes and collections are part of the UCT Biobank:
- Dr. Senckenberg Institute of Pathology (Senckenberg Biobank SBB)
- Edinger Institute (Neuropathology)
- Department of Medicine 2, Hematology/Medical Oncology
- Department of Dermatology
- Department of Pediatric and Adolescent Medicine
- Organoid Collection at the Georg-Speyer-Haus (GSH)
Clinical data for scientific purposes can be provided via the UCT Tumor Documentation, which was established in 2012. This centralized unit documents the clinical course of all cancer patients treated at the UCT. Information on tumor diagnosis, treatment and survival of the patient as well as entity specific parameters (e.g. molecular markers) are recorded in a structured manner.
Both data from the tumor documentation and patient samples collected in the biobank are available to scientists for oncological research on request using our online submission software APProVe.
The UCT Biobank and Tumor Documentation are part of the interdisciplinary Biobank and Database Frankfurt (iBDF), a network of local biobanks and data collections at the University Hospital Frankfurt. Collections within the iBDF work with a standardized application and approval process and a central IT infrastructure (CentraXX) for sample administration and annotation. Thanks to a clinic-wide, disease-independent patient consent (Broad Consent), samples and data from clinical routine can be made available to researchers in a standardized application procedure.

Sample and Data Collection and Availability
The collected samples of the UCT Biobank are either residual tissue samples, which are sent to the pathologies as part of surgical interventions, or liquid samples (blood, bone marrow, urine, stool...), which are collected in clinical routine and diagnostics without additional intervention for the tumor patients.
Tissue samples are stored as cryopreserved fresh material in liquid nitrogen immediately after completion of all relevant diagnostic tests. The Dr. Senckenberg Insitute of Pathology and the Edinger Institute (Neuropathology) are the main institutes collecting these kinds of samples. If possible, tumor tissue as well as adjacent normal tissue is preserved. In addition, the formalin-fixed paraffin-embedded (FFPE) tissue blocks from the pathology archives are available for scientific projects. These also form the basis for Tissue Micro Arrays (TMA), which are available for different tumor entities.
Additionally, tissue samples from colon cancer patients are routinely cultured into colon cancer organoids. These are processed, stored and characterized at the Georg-Speyer-Haus and are also available for further research.
In case of haemato-oncological diseases, mononuclear cells (MNCs) are routinely isolated from blood, bone marrow or leukapheresis products and stored in liquid nitrogen.
Project specific derivatives (e.g. DNA, RNA or protein extracts) can be prepared from all collected primary materials. Project-specific collection of other body fluids (e.g. urine, stool, plasma, serum) or (immuno-)histological staining is also possible on request.
In general, all samples are provided with a basic set of clinical parameters (Basisdatensatz) but additional data can delivered upon request (see also Clinical Data).
How to get patient samples and clinical data for scientific purposes
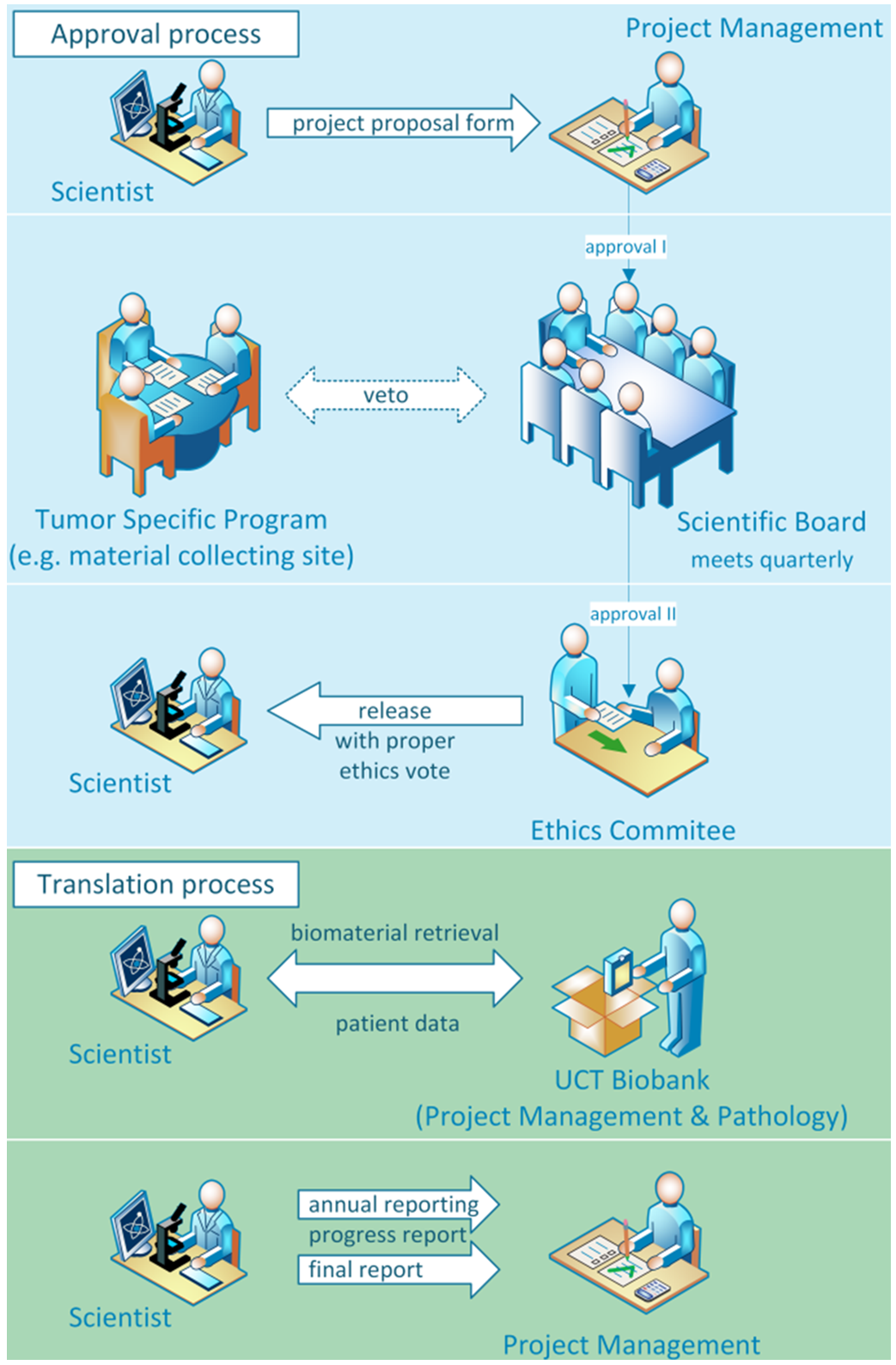
Usage of the collected samples and clinical data for oncological research is only possible upon request and with a final approval of all parties involved. To this end, a project proposal must be submitted to the UCT Biobank's project management team. The following is a small guide on how to get samples and clinical data from the UCT Biobank and tumor documentation for scientific research purposes.
1. Submit project proposal
Research project proposals are submitted online using APProVe. With APProVe, interested investigators can apply for patient samples and/or clinical data online and can see the current status of all their UCT projects.
Please note the submission deadlines for the meetings of the oncological Scientific Board (see 3.)!
2. Internal review (for completeness and feasibility)
All applications will be checked for completeness and basic feasibility by the project management of the UCT Biobank. If there are any questions, we will get back to you!
3. Review and evaluation by the Scientific Board
Before the requested samples and/or clinical data are provided, the submitted proposals are reviewed by a scientific committee, the Scientific Board, and afterwards evaluated by the local ethics committee.
In quarterly meetings, central and independent review, evaluation, and approval of all oncological project proposals take place. The Scientific Board consists of appointed members from all tumor-specific teams and cross-sectional disciplines involved in the collection of samples and clinical data. A decision is made on pre-defined criteria.
The oncological Scientific Board meets quarterly to review and evaluate the submitted project proposals, which must be submitted at least 2 weeks before the meeting.
| Submission deadline for oncological proposals | Scientific Board meeting |
| November 18, 2024 | December 2, 2024 |
| February 17, 2025 | March 3, 2025 |
| May 12, 2025 | May 26, 2025 |
| August 18, 2025 | September 1, 2025 |
| November 17, 2025 | December 1, 2025 |
For more information please contact Dr. Kristina Götze (Phone 069 / 6301-87187, kristina.goetze@unimedizin-ffm.de).
4. Project specific ethic votes in a simplified procedure
Following approval by the Scientific Boards, the project management team submits the forms to the local Ethics Committee, which evaluates proposals of the UCT Biobank in a simplified procedure (without meeting).
5. Provision of samples and clinical data
Requested patient samples and/or clinical oncology-related data will only be allocated after approval of both the Scientific Board and the Ethics Committee.
Samples are provided together with a basic data set containing the most important clinical information. Further datasets are available, if the scope and extent is predefined in the project proposals.
Data sets for projects using solely clinical data will only be provided subject to prior consultation and definition of the required data elements.
6. Reporting and publications
The project management team of the UCT Biobank and Tumor Documentation has to be informed about the progress of the project in an annual interim report.
Publications based on samples and/or clinical data that were provided by the UCT Biobank and Tumor Documentation, the origin of the samples and data including the corresponding project number must be stated as follows:
Tissue/tumor samples and/or patient data used in this study were provided by the University Cancer Center Frankfurt (UCT). Written informed consent was obtained from all patients and the study was approved by the institutional Review Boards of the UCT and the Ethical Committee at the University Hospital Frankfurt (project-number: XXX-YY-20YY).
Project completion as well as all resulting publications have to be reported immediately to the project management of the UCT Biobank and Tumor Documentation.
7. Feasibility and availability queries and cohort compilation with CentraXX
The UCT project and data management team will advise on project feasibility and availability of samples types, numbers and data sets.
Additionally, the feasibility of a project can also be assessed in advance using the central database (CentraXX) of the iBDF/UCT Biobank and Tumor Documentation. Scientists at the University Hospital Frankfurt can gain a pseudonymized access to CentraXX in accordance with data protection laws. Here, information on collected samples as well as clinical, diagnostic and scientific data is linked on a patient-related basis and can be queried and viewed for feasibility studies or the compilation of research cohorts.